Interventional Care

We notice that you are visiting us from . This site only services US-based visitors. Would you like to visit the site that is appropriate for your location?




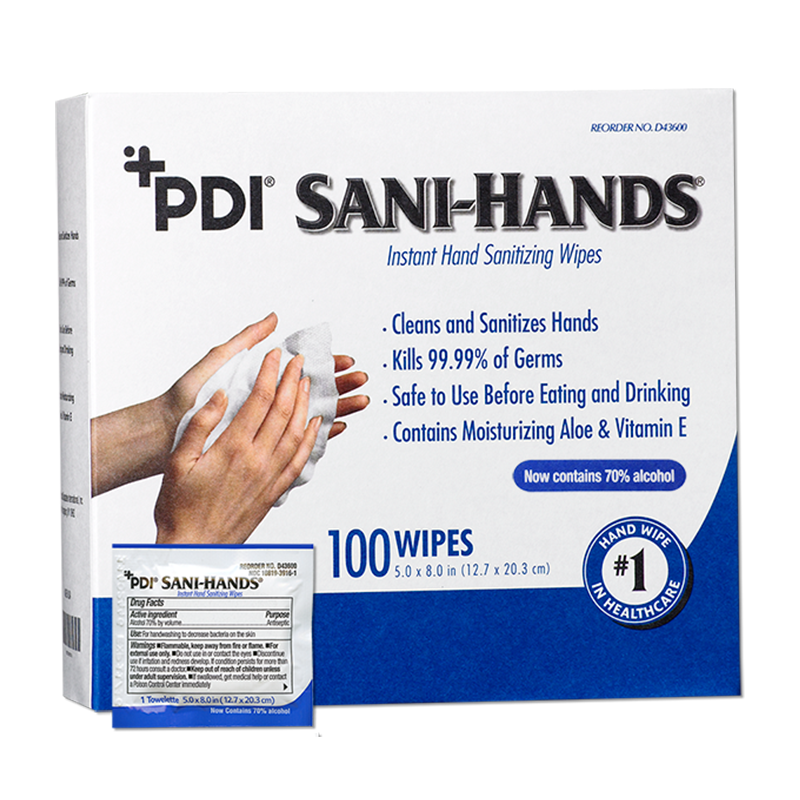





An ideal hand hygiene solution for staff, visitors, patients or residents who cannot get out of bed to clean their hands. The cost to treat an HAI can be as much as $58,500. By preventing just one, a patient hand hygiene program could pay for itself for an entire year.1
Accessories & Compliance Tools
This instructions for use sign can be placed around the facility to encourage proper hand hygiene technique with…
Small sign with icons to reinforce sanitizing hands before and after eating. Place on the bedside table to…
Did you know that our Sani-Hands® wipes help to remove bacteria more effectively than gels and foams from…
When running from patient to patient, it’s hard to always remember or find time to perform hand hygiene.…
Healthcare professionals work overtime to help save lives every day, and sometimes these jobs mean getting their hands…
Engage your patients in their care with this Sani-Hands video, which covers the importance of hand hygiene and…
Influenza (flu) is a highly contagious respiratory illness caused by influenza viruses. It can cause mild to severe…
The Sani-Hands Clinical Compendium provides a complete guide to efficacy data, clinical studies and safety information for the…
Patient hand hygiene is an important element of any infection prevention program. This “Patient Hand Hygiene” video series…
Patient hand hygiene is an important element of any infection prevention program. This “Patient Hand Hygiene” video series…
Patient hand hygiene is an important element of any infection prevention program. This “Patient Hand Hygiene” video series…
Patient hand hygiene is an important element of any infection prevention program. This “Patient Hand Hygiene” video series…
Patient hand hygiene is an important element of any infection prevention program. This “Patient Hand Hygiene” video series…
A downloadable listing of PDI’s point of care accessories for Sani-Cloth® and Sani-Hands® products, including 3-in-1 Wall Bracket®,…
Sani-Hands® Instant Hand Sanitizing Wipes are an ideal hand hygiene solution for staff, visitors, patients, or residents who…
PDI offers a broad range of evidence-based, market-leading Interventional Care, Environment of Care, and Patient Care solutions, all…
Are Sani-Hands® Instant Hand Sanitizing Wipes an FDA regulated product?
Sani-Hands Instant Hand Sanitizing Wipes are regulated by the US FDA as an OTC (Over-the-Counter) drug product covered under the Tentative Final Monograph for Healthcare Antiseptic Drug Products.
How long should I apply Sani-Hands® Instant Hand Sanitizing Wipes to the hands?
A contact time of 15 seconds provides a 99.99% reduction.
What is the expiration date on Sani-Hands® Instant Hand Sanitizing Wipes?
Sani-Hands wipes have a 24 month expiration date from the date of finished product manufacturing.
Some PDI products state "store at room temperature." What is the definition of room temperature?
For our EPA-regulated products, such as Sani-Cloth® and Sani-Prime™ brand products, room temperature is defined as an average temperature of 25◦ C (77◦ F) and within a temperature range of 15◦ C to 30◦ C (59◦ F to 86◦ F). For our FDA-regulated products, such as Prevantics® brand products, “controlled room temperature” indicates a temperature maintained thermostatically that encompasses the usual customary working environment of 20◦ C to 25◦ C (68◦ F to 77◦ F). SOURCE: USP 41-NF 36 General Notices and Requirements (August 1, 2013 First Supplements) Section 10.30.50. “Room Temperature” indicates the temperature prevailing in a working area. Section 10.30.60. Controlled Room Temperature
SKU # P013500
10/case
SKU # P58500
10/case
SKU # P010900
10/case
SKU # Q52510
10/case
SKU # P010600
1 unit per case
SKU # P015300
1 units per case
SKU # P013600
1 unit per case
SKU # P013700
1 per case
SKU # P014100
1 unit per case