Interventional Care

We notice that you are visiting us from . This site only services US-based visitors. Would you like to visit the site that is appropriate for your location?
A new positive blood culture appears on your surveillance. And it appears to be a commensal organism. Or is it? Any Infection Preventionist will admit to having these challenges daily. Blood culture contamination is a problematic issue in healthcare. Not only will a contaminant increase the likelihood of inappropriate & unnecessary antibiotic therapy, it will also potentially add a significant healthcare cost— either to the hospital or to the patient. Avoiding these outcomes is the goal!
What does the research say about blood culture contamination & is blood culture contamination really an issue?
Yes, according to literature, 20% to 50% are likely false positives.¹ Blood culture contamination rates should not exceed the recommended 3% of all blood culture collections according to the American Society for Microbiology (ASM) and the Clinical Laboratory Standards Institute (CLSI).¹
Contaminated blood cultures add an exorbitant financial burden to health care organizations due to the additional therapies, testing and increased patient lengths of stay. According to some studies, the cost for a false positive blood cultures can be between $4,500 and $8,720 per patient.² The toll that false positive blood cultures takes on the patient is substantial. A false positive is not always easily distinguishable from a true infection. Many providers will err on the side of caution and treat the false positive with antibiotics. Unnecessary antibiotics can increase a patient’s risk for other negative outcomes including infections such as Clostridium difficile and allergic reactions to antibiotics.²
What are the guidelines and some strategies that healthcare workers can take to prevent blood culture contamination?
Evidence supports several effective strategies that will assist in reducing contamination of blood cultures. These strategies include standardized blood culture collection kits, phlebotomy teams, and drawing from a vein (venipuncture) vs from a catheter.¹
Skin antisepsis prior to blood culture collection is repeatedly highlighted in evidence based literature. It is essential that skin antisepsis is performed correctly before any skin injection including venipuncture. According to the Cumitech guidelines for blood cultures from ASM, “failure to adequately cleanse the skin using meticulous technique and an appropriate antiseptic agent increases the risk that microbial flora of the skin, such as coagulase-negative staphylococci or Corynebacterium spp., will contaminate the blood culture.”³ These guidelines also note that prepping of the venipuncture site should be done by “vigorously cleansing for 30 seconds back and forth across the site” with alcohol and chlorhexidine, then allowing to dry for at least 30 seconds prior to needle insertion.³ Using an alcoholic chlorhexidine skin antiseptic for skin prep is also recommended by SHEA/IDSA and CDC in their guidelines to prevent intravascular bloodstream infections.⁴ ⁵
Prevantics® skin swabsticks, which are ideal for skin antisepsis for blood culture collection due to their premoistened double sided applicator, now match the Cumitech guidelines with a 30 second prep and a 30 second dry time.
A new study in the Journal of Vascular Access highlighted the importance of using Chlorhexidine gluconate (CHG) / alcohol for skin antisepsis to reduce blood culture contamination rates in the Emergency Department. They experienced a statistically significant drop in blood culture contamination rates (from 4.5% to 1.5%).² The rates remained low at 1.9% the following month.² This success was a result of replacing alcohol swabs with Prevantics CHG/ Isopropyl alcohol (IPA) swabs in procedural trays and in bedside carts.
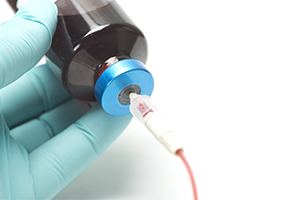
Staff were also able to use the CHG/ IPA swabs for cleaning the blood culture bottle tops (septum).² According to clinical practice guidelines for prevention of blood culture contamination from the Emergency Nurses Association (ENA), blood culture bottle tops sterility varies by manufacturer. They recommend to clean the blood culture bottle tops and noted a study that showed a significant reduction in contamination rates when cleaning blood culture bottles with an antiseptic prior to specimen inoculation.⁶ Prevantics Device Swab & Strip can be used for disinfection of the blood culture bottle tops as well.
It is critical that facilities evaluate and monitor their blood culture contamination rates routinely. Incorporating evidence based reduction strategies to reduce blood culture contamination is key to increasing patient safety and good outcomes.
¹Snyder SR, Favoretto AM, Baetz RA, et al. Effectiveness of practices to reduce blood culture contamination: A Laboratory Medicine Best Practices systematic review and meta-analysis. Clinical biochemistry. 2012; 45(0):999-1011. doi:10.1016/j.clinbiochem.2012.06.007.
²Ryan, C. (2017). Implementation of the Theory of Planned Behavior to Promote Compliance with a Chlorhexidine Gluconate Protocol. The Journal of the Association for Vascular Access, 22(2), 64-70.
³Baron, E. J., M. P. Weinstein, W. M. Dunne, Jr., P. Yagupsky, D. F. Welch, and D. M. Wilson.2005. Cumitech 1C, Blood Cultures IV. Coordinating ed., E. J. Baron. ASM Press, Washington, D.C.
⁴Miller, D. L., & O’Grady, N. P. (2012). Guidelines for the prevention of intravascular catheter-related infections: recommendations relevant to interventional radiology for venous catheter placement and maintenance. Journal of vascular and interventional radiology: JVIR, 23(8), 997. https://www.cdc.gov/infectioncontrol/guidelines/bsi/index.html
⁵Marschall, J., Mermel, L., Fakih, M., Hadaway, L., Kallen, A., O’Grady, N., Yokoe, D. (2014). Strategies to Prevent Central Line-Associated Bloodstream Infections in Acute Care Hospitals: 2014 Update. Infection Control & Hospital Epidemiology, 35(S2), S89-S107. doi:10.1017/S0899823X00193870
⁶Emergency Nurses Association. (2016). Clinical practice guideline: prevention of blood culture contamination. https://www.ena.org/docs/default-source/resource-library/practice-resources/cpg/bcccpg2c37f1815b664d2fa8d7e9fd0f475a41.pdf?sfvrsn=6d1899fb_8