Interventional Care

We notice that you are visiting us from . This site only services US-based visitors. Would you like to visit the site that is appropriate for your location?










Get ready for Sani-HP1, our latest* hydrogen peroxide-based disinfectant, designed to tackle harmful pathogens like Norovirus, Candida auris, multi-drug resistant bacteria, and TB in just one minute. With broad-spectrum efficacy and a Category IV Toxicity Profile, Sani-HP1 delivers proven efficacy while being compatible with healthcare surfaces1.
Sani-HP1 will feature PDI’s proprietary Hydroguard Technology™, designed to disinfect without compromising surface compatibility. Sustainable and versatile, Sani-HP1’s active ingredient breaks down into water and oxygen and will be available in various packaging formats to help meet your facility’s environmental goals.
Stay tuned for updates to be among the first to experience this rapid, effective disinfection.
*Sani-HP1 is not yet available for sale or distribution into Hawaii or Oregon—contact your PDI rep for more information!
SELECT A SIZE
9480-17
Intermediate-level disinfectant
12 months
Daily, everyday disinfection
Category IV (no PPE required)
1 minute
23 Vlaims including Bactericidal, Virucidal, Fungicidal, Tuberculocidal including TB, C. auris, and Norovirus
Hydrogen peroxide-based formula
Engineered for superior compatibility with materials commonly found in healthcare settings, utilizing Hydroguard Technology™ to outperform leading hydrogen peroxide-based products3
Sani-Hands® Instant Hand Sanitizing Wipes are an ideal hand hygiene solution for staff, visitors, patients, or residents who…
Did you know that our Sani-Hands® wipes help to remove bacteria more effectively than gels and foams from…
The Dual Access Lid is a breakthrough in canister packaging design, based on the vital need for quicker…
Do the PDI Sani-Cloth® products kill the Delta variant of SARS-CoV-2?
The EPA guidance states: “EPA expects all products on List N to kill all strains of SARS-CoV-2. Genetic changes to the virus do not impact the efficacy of disinfectants. List N disinfectants work by chemically inactivating viruses. The difficulty of killing a virus depends on its physical features, and the recent mutations to SARS-CoV-2 have not changed the basic physical properties.”
The PDI Sani-Cloth products that are on List N and have a specific claim for SARS-CoV-2 are:
Can PDI Germicidal Wipes and Sprays be used on toys?
PDI disinfectants are available in EPA-Registered formulations that are approved and labeled for use on hard, non-porous toys. The products clean and disinfect in a one-step process, unless visibly soiled. Once disinfected, toys should be rinsed with potable water (tap water) to remove any residue and allowed to air dry. According to the Association for Professionals in Infection Control and Epidemiology (APIC), the recommended procedure for disinfecting toys is “Toys should be cleaned/disinfected between patients, especially those that are visibly soiled, mouthed, or used by patients in isolation. Toys should be washed thoroughly; disinfected with a non-toxic, low-level disinfectant, and air-dried completely.” Infection control experts recommend only washable toys for sharing. Stuffed animals and toys that cannot be cleaned and disinfected should not be shared.
SOURCE: APIC Text of Infection Control and Epidemiology, Chapter 39, p. 14-15, Association for Professionals in Infection Control and Epidemiology, 2011. www.apic.org
Do PDI Germicidal Wipes and Sprays contain any ingredient listed as carcinogenic?
PDI disinfectants DO NOT contain any ingredients listed as carcinogenic by the National Toxicology Program (NTP), American Conference of Governmental Industrial Hygienists (ACGIH), and Occupational Safety and Health Administration (OSHA). This applies to ALL Sani-Cloth products, including Sani-24, Sani-HyPerCide, Sani-Cloth Prime, Sani-Prime, Super Sani-Cloth, Sani-Cloth AF3, Sani-Cloth Bleach, Sani-Cloth Plus, and Sani-Cloth HB brands. To register any disinfectant product with the US Environmental Protection Agency (EPA), the manufacturer is required to provide the EPA with the product’s manufacturing process, active and inactive ingredients, efficacy, chemistry, toxicity, and information about relevant impurities. The EPA conducts a thorough review of these materials and product ingredients. The agency would not register any product if it contained carcinogens without requiring relevant label warnings (40 CFR 156.10(g)(7)). As such, Sani-Cloth Wipes and Sani-Prime Spray do not contain carcinogenic label warnings.
Is Sani-HyPerCide® Germicidal Disinfectants a Sporicidal formulation?
Yes. Sani-HyPerCide® is the first ready-to-use Hydrogen Peroxide formula that is effective against Clostridioides difficile.
What personal protective equipment (PPE) is required when using ani-HyPerCide™ Germicidal Spray and Wipes?
For all PDI products, according to the label instruction, the use of gloves or other PPE is not required to handle the product, particularly in non-clinical settings. Therefore, the routine use of PPE is not required unless potentially infectious blood or bodily fluids are present. If bloodborne pathogens are present, such as HIV, HBV, and HCV, follow label instructions. You should, however, wear PPE as appropriate in accordance with your facility protocol. Also, in compliance with good industrial and health hygiene, you should wear gloves when cleaning and disinfecting in a patient setting.
Date of Disclosure: 3/3/2025
| Ingredients | CAS Numbers | Function | Designated List(s) |
|---|---|---|---|
| Water | 7732-18-5 | Diluent | None |
| Benzyl Alcohol | 100-51-6 | Solvent | EU Fragrance Allergens |
| Hydrogen Peroxide | 7722-84-1 | Antimicrobial Agent | None |
| Sodium Hydroxide | 1310-73-2 | pH Adjuster | OEHHA REL |
| Phosphoric Acid | 7664-38-2 | pH Adjuster | OEHHA REL |
| Anionic Surfactant | Withheld for CBI | Surfactant | None |
| C10-12 Alcohol Ethoxylates | 66455-15-0 | Surfactant | None |
| Organic Acid | Withheld for CBI | Chelating Agent | None |
Lorem ipsum dolor sit amet, consectetur adipiscing elit. Maecenas scelerisque turpis nec massa sodales, et laoreet ex fermentum. Suspendisse potenti. Orci varius natoque penatibus et magnis dis parturient montes, nascetur ridiculus mus.
| SKU Number | Product Size | Dimensions | Packaging | GTIN | Country of Manufacturer |
|---|---|---|---|---|---|
| P62572 | Medium Canister | 6" x 5" | 12/275s | 00000 | USA |
| P42672 | Large Canister | 6" x 9" | 12/165s | 00000 | USA |
| P83184 | X-Large Canister | 7.5" x 15" | 6/95s | 00000 | USA |
| P0125P | Bulk Packaging | 11" x 12" | 2/300s | 00000 | USA |
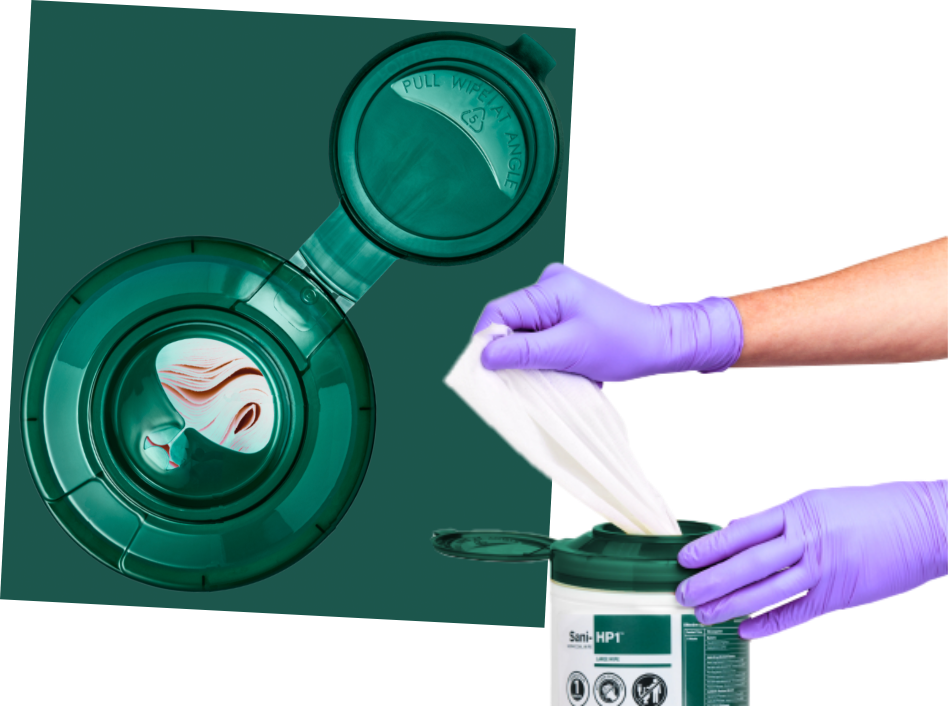


Interested in upgrading your disinfection practices? Get in touch with one of our team members today.
1on hard nonporous surfaces.
2In comparison to the weight of plastic for similar count canister or pail format.
3Based on internal testing. Data on file (ELN 20230126-002 & Report 20230126-002)